A study conducted by the US Centers for Disease Control and Food and Drug Administration has shown that the risk of myocarditis following mRNA COVID vaccination is around 133x greater than the background risk in the population.
This means Covid vaccination increases the risk of suffering myocarditis, an autoimmune disease causing inflammation of the heart, by 13,200%.
The study, conducted by researchers from the U.S. Centers for Disease Control (CDC) as well as from several U.S. universities and hospitals, examined the effects of vaccination with products manufactured by Pfizer-BioNTech and Moderna.
The study’s authors used data obtained from the CDC’s VAERS reporting system which were cross-checked to ensure they complied with CDC’s definition of myocarditis; they also noted that given the passive nature of the VAERS system, the number of reported incidents is likely to be an underestimate of the extent of the phenomenon.
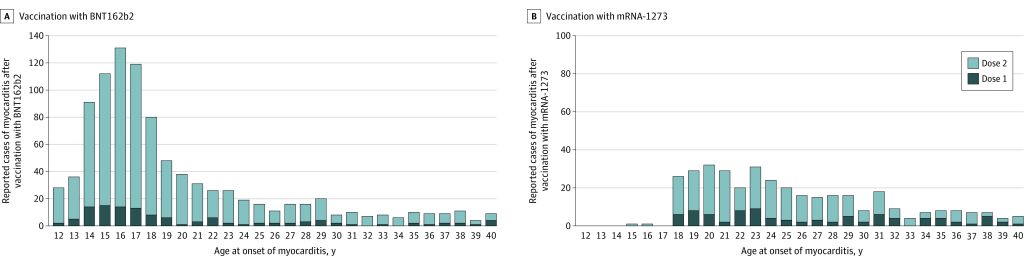
1626 cases of myocarditis were studied, and the results showed that the Pfizer-BioNTech product was most associated with higher risk, with 105.9 cases per million doses after the second vaccine shot in the 16 to 17 age group for males, and 70.7 cases per million doses after the second shot in the 12 to 15 age group for males. The 18 to 24 male age group also saw significantly higher rates of myocarditis for both Pfizer’s and Moderna’s products (52.4 and 56.3 cases per million respectively)
Key Points
Question What is the risk of myocarditis after mRNA-based COVID-19 vaccination in the US?
Findings In this descriptive study of 1626 cases of myocarditis in a national passive reporting system, the crude reporting rates within 7 days after vaccination exceeded the expected rates across multiple age and sex strata. The rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).
Meaning Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men.
Key Points
Question What is the risk of myocarditis after mRNA-based COVID-19 vaccination in the US?
Findings In this descriptive study of 1626 cases of myocarditis in a national passive reporting system, the crude reporting rates within 7 days after vaccination exceeded the expected rates across multiple age and sex strata. The rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).
Meaning Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men.
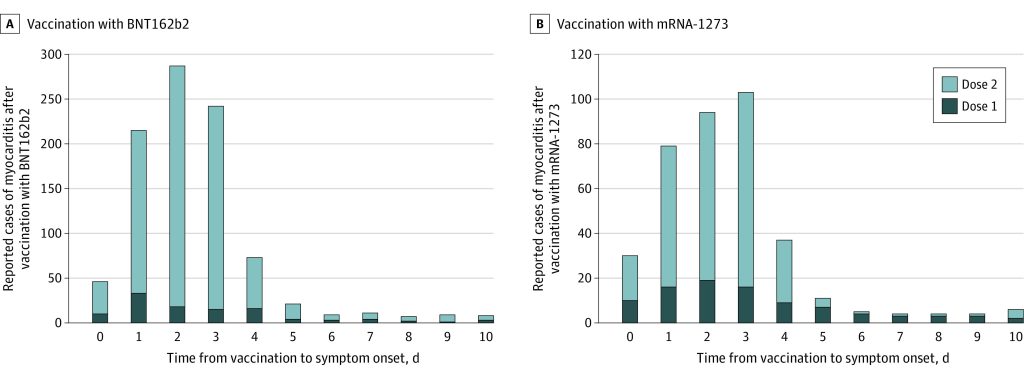
The study found that the median time to symptom onset was two days, and that 82 percent of cases were in males, consistent with previous studies. Around 96 percent of affected people were hospitalised, with most treated with nonsteroidal anti-inflammatory drugs; 87 percent of those hospitalised had resolution of symptoms by time of discharge.





Recent Comments