Report Conclusions and Relevance. Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men. This risk should be considered in the context of the benefits of COVID-19 vaccination.
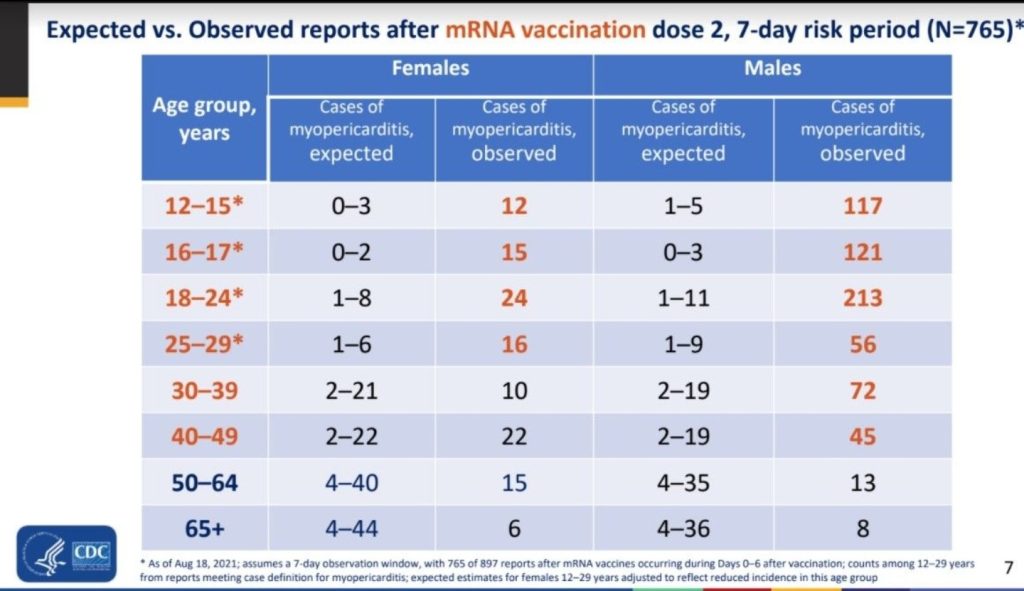
Between December 14, 2020, and August 31, 2021, 192 405 448 individuals older than 12 years of age received a total of 354 100 845 mRNA-based COVID-19 vaccines. VAERS received 1991 reports of myocarditis (391 of which also included pericarditis) after receipt of at least 1 dose of mRNA-based COVID-19 vaccine and 684 reports of pericarditis without the presence of myocarditis.
Of the 1991 reports of myocarditis, 1626 met the CDC’s case definition for probable or confirmed myocarditis. There were 208 reports that did not meet the CDC’s case definition for myocarditis and 157 reports that required more information to perform adjudication . Of the 1626 reports that met the CDC’s case definition for myocarditis, 1195 (73%) were younger than 30 years of age, 543 (33%) were younger than 18 years of age, and the median age was 21 years (IQR, 16-31 years) . Of the reports of myocarditis with dose information, 82% (1265/1538) occurred after the second vaccination dose. Of those with a reported dose and time to symptom onset, the median time from vaccination to symptom onset was 3 days (IQR, 1-8 days) after the first vaccination dose and 74% (187/254) of myocarditis events occurred within 7 days. After the second vaccination dose, the median time to symptom onset was 2 days (IQR, 1-3 days) and 90% (1081/1199) of myocarditis events occurred within 7 days.
Males comprised 82% (1334/1625) of the cases of myocarditis for whom sex was reported. The largest proportions of cases of myocarditis were among White persons (non-Hispanic or ethnicity not reported; 69% [914/1330]) and Hispanic persons (of all races; 17% [228/1330]). Among persons younger than 30 years of age, there were no confirmed cases of myocarditis in those who died after mRNA-based COVID-19 vaccination without another identifiable cause and there was 1 probable case of myocarditis but there was insufficient information available for a thorough investigation. At the time of data review, there were 2 reports of death in persons younger than 30 years of age with potential myocarditis that remain under investigation and are not included in the case counts.

READ ENTIRE REPORT HERE: https://jamanetwork.com/journals/jama/fullarticle/2788346
Top 13 Studies Evincing Myocarditis Is Worse from the COVID ‘Vaccines’ Than from COVID-19 Itself for Those Under 40
Here are the top 13 studies showing that the odds of developing myocarditis from the “vaccines” are greater than the odds of developing myocarditis from COVID-19 for those under age 40. This post also includes a list of anecdotal stories showcasing how detrimental vaccine-induced myocarditis is on young people.
NOTE: Direct quote from the COMIRNATY Summary Basis for Regulatory Action: “Post-EUA safety surveillance reports received by FDA and CDC identified serious risks for myocarditis and pericarditis following administration of COMIRNATY. Reporting rates for medical chart-confirmed myocarditis/pericarditis in VAERS have been higher among males under 40 years of age than among females and older males and have been highest in males 12-17 years of age (65 cases per million doses administered as per CDC communication on August 20, 2021), particularly following the second dose, and onset of symptoms within 7 days following vaccination. Although some cases of vaccine associated myocarditis/pericarditis required intensive care support, available data from short-term follow up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae and outcomes in affected individuals. A mechanism of action by which the vaccine could cause myocarditis and pericarditis has not been established.”
1. Medical Archive (Not Yet Published in Peer-Reviewed Journal) Dated December 21, 2021
TITLE: Risk of myocarditis following sequential COVID-19 vaccinations by age and sex
METHODS: “In brief, we used the NHS Immunisation Management Service (NIMS) database, which includes
data for all people receiving a COVID-19 vaccine in England. We linked individual patient data to national data for hospital admission, mortality and SARS-CoV-2 testing to examine associations between exposures to the first, second or third dose of ChAdOx1, BNT162b2 or mRNA-1273 vaccine, or a positive SARS-CoV-2 test before or after vaccination, and hospital admission or death from myocarditis. The self-controlled case series (SCCS) method [13, 14] compares the incidence rate of myocarditis in exposed and unexposed periods within individuals implicitly
controlling for within person covariates. The incidence rate ratio (IRR) is calculated for hospital admission or death in a 1-28 day risk period after vaccination or a positive test, compared to baseline periods. The IRR was calculated following stratification by sex and age in those younger or older than 40 years.”
CONCLUSION: In summary, the risk of hospital admission or death from myocarditis is greater following
COVID-19 infection than following vaccination and remains modest following sequential doses
of mRNA vaccine including a third booster dose of BNT162b in the overall population. However,
the risk of myocarditis following vaccination is consistently higher in younger males, particularly
following a second dose of RNA mRNA-1273 vaccine.
FIGURES:


2. Medical Archive (Not Yet Published in Peer-Reviewed Journal) Dated August 30, 2021
TITLE: SARS-CoV-2 mRNA Vaccination-Associated Myocarditis in Children Ages 12-17: A Stratified National Database Analysis
METHODS: “Using the Vaccine Adverse Event Reporting System (VAERS), this retrospective epidemiological assessment reviewed reports filed between January 1, 2021, and June 18, 2021, among adolescents ages 12-17 who received mRNA vaccination against COVID-19. Symptom search criteria included the words chest pain, myocarditis,
pericarditis and myo/pericarditis to identify children with evidence of cardiac injury. The word troponin was a required element in the laboratory findings. Inclusion criteria were aligned with the CDC working case definition for probable myocarditis. Stratified cardiac adverse event (CAE) rates were reported for age, sex and vaccination dose number. A harm-benefit analysis was conducted using existing literature on COVID-19-related hospitalization risks in this demographic.”
CONCLUSION: “A total of 257 CAEs [cardiac adverse events] were identified. Rates per million following dose 2 among males were 162.2 (ages 12-15) and 94.0 (ages 16-17); among females, rates were 13.0 and 13.4 per million, respectively. For boys 12-15 without medical comorbidities receiving their second mRNA vaccination dose, the rate of CAE is 3.7 to 6.1 times higher than their 120-day COVID19 hospitalization risk as of August 21, 2021 (7-day hospitalizations 1.5/100k population) and 2.6-4.3-fold higher at times of high weekly hospitalization risk (7-day hospitalizations 2.1/100k), such as during January 2021. For boys 16-17 without medical comorbidities, the rate of CAE is currently 2.1 to 3.5 times higher than their 120-day COVID-19 hospitalization risk, and 1.5 to 2.5 times higher at times of high weekly COVID-19 hospitalization.
Post-vaccination CAE rate was highest in young boys aged 12-15 following dose two. For boys 12-17 without medical comorbidities, the likelihood of post vaccination dose two CAE is 162.2 and 94.0/million respectively. This incidence exceeds their expected 120-day COVID-19 hospitalization rate at both moderate (August 21, 2021 rates) and high COVID-19 hospitalization incidence. Further research into the severity and long-term sequelae of post vaccination CAE is warranted. Quantification of the benefits of the second vaccination dose and vaccination in addition to natural immunity in this demographic may be indicated to minimize harm.”
3. JAMA Cardiology (Published June 29, 2021)
TITLE: Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military
METHODS: “This retrospective case series studied patients within the US Military Health System who experienced myocarditis after COVID-19 vaccination between January and April 2021. Patients who sought care for chest pain following COVID-19 vaccination and were subsequently diagnosed with clinical myocarditis were included.”
CONCLUSION: “A total of 23 male patients (22 currently serving in the military and 1 retiree; median [range] age, 25 [20-51] years) presented with acute onset of marked chest pain within 4 days after receipt of an mRNA COVID-19 vaccine. All military members were previously healthy with a high level of fitness. Seven received the BNT162b2-mRNA vaccine and 16 received the mRNA-1273 vaccine. A total of 20 patients had symptom onset following the second dose of an appropriately spaced 2-dose series. All patients had significantly elevated cardiac troponin levels. Among 8 patients who underwent cardiac magnetic resonance imaging within the acute phase of illness, all had findings consistent with the clinical diagnosis of myocarditis. Additional testing did not identify other etiologies for myocarditis, including acute COVID-19 and other infections, ischemic injury, or underlying autoimmune conditions. All patients received brief supportive care and were recovered or recovering at the time of this report. The military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period. While the observed number of myocarditis cases was small, the number was higher than expected among male military members after a second vaccine dose.
In this case series, myocarditis occurred in previously healthy military patients with similar clinical presentations following receipt of an mRNA COVID-19 vaccine. Further surveillance and evaluation of this adverse event following immunization is warranted. Potential for rare vaccine-related adverse events must be considered in the context of the well-established risk of morbidity, including cardiac injury, following COVID-19 infection.“
4. The New England Journal of Medicine (Published October 6, 2021)
TITLE: Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel
METHODS: “We retrospectively reviewed data obtained from December 20, 2020, to May 31, 2021, regarding all cases of myocarditis and categorized the information using the Brighton Collaboration definition. We analyzed the occurrence of myocarditis by computing the risk difference for the comparison of the incidence after the first and second vaccine doses (21 days apart); by calculating the standardized incidence ratio of the observed-to-expected incidence within 21 days after the first dose and 30 days after the second dose, independent of certainty of diagnosis; and by calculating the rate ratio 30 days after the second dose as compared with unvaccinated persons.”
CONCLUSION: “Among 304 persons with symptoms of myocarditis, 21 had received an alternative diagnosis. Of the remaining 283 cases, 142 occurred after receipt of the BNT162b2 vaccine; of these cases, 136 diagnoses were definitive or probable. The clinical presentation was judged to be mild in 129 recipients (95%); one fulminant case was fatal. The overall risk difference between the first and second doses was 1.76 per 100,000 persons (95% confidence interval [CI], 1.33 to 2.19), with the largest difference among male recipients between the ages of 16 and 19 years (difference, 13.73 per 100,000 persons; 95% CI, 8.11 to 19.46). As compared with the expected incidence based on historical data, the standardized incidence ratio was 5.34 (95% CI, 4.48 to 6.40) and was highest after the second dose in male recipients between the ages of 16 and 19 years (13.60; 95% CI, 9.30 to 19.20). The rate ratio 30 days after the second vaccine dose in fully vaccinated recipients, as compared with unvaccinated persons, was 2.35 (95% CI, 1.10 to 5.02); the rate ratio was again highest in male recipients between the ages of 16 and 19 years (8.96; 95% CI, 4.50 to 17.83), with a ratio of 1 in 6637.
The incidence of myocarditis, although low, increased after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients. The clinical presentation of myocarditis after vaccination was usually mild.”
5. Journal: Journal of American Medical Association (Published August 4, 2021)
TITLE: Myocarditis and Pericarditis After Vaccination for COVID-19
METHODS: “Forty hospitals in Washington, Oregon, Montana, and Los Angeles County, California, that were part of the Providence health care system and used the same electronic medical record (EMR) were included. All patients with documented COVID-19 vaccinations administered inside the system or recorded in state registries at any time through May 25, 2021, were identified. Vaccinated patients who subsequently had emergency department or inpatient encounters with diagnoses of myocarditis, myopericarditis, or pericarditis were ascertained from EMRs (see eTables 1 and 2 in the Supplement for exclusions and definitions).
The monthly rates of first-time hospital diagnoses (excluding patients with previous diagnoses in January 2018–January 2019) in January 2019 through January 2021 (prevaccine period) and February through May 2021 (vaccine period) were compared.”
CONCLUSION:
“Two distinct self-limited syndromes, myocarditis and pericarditis, were observed after COVID-19 vaccination. Myocarditis developed rapidly in younger patients, mostly after the second vaccination.Pericarditis affected older patients later, after either the first or second dose.
Some vaccines are associated with myocarditis, including mRNA vaccines, and the Centers for Disease Control and Prevention recently reported a possible association between COVID-19 mRNA vaccines and myocarditis, primarily in younger male individuals within a few days after the second vaccination, at an incidence of about 4.8 cases per 1 million. This study shows a similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting. Additionally, pericarditis may be more common than myocarditis among older patients.”
7. Journal: The Journal of Pediatrics (Published March 26, 2022)
TITLE: Persistent Cardiac MRI Findings in a Cohort of Adolescents with post COVID-19 mRNA vaccine myopericarditis
METHODS: “This case review includes patients younger than 18 years of age presenting to Seattle Children’s Hospital with chest pain and elevated serum troponin level from April 1, 2021 to January 7, 2022 within one week of receiving the second dose of the Pfizer COVID-19 mRNA vaccine. Institutional Review Board approval was obtained. All patients were evaluated by a pediatric cardiologist, underwent ECG and echocardiogram, and were admitted for observation with telemetry, serial troponin measurements, and repeat cardiac testing as needed. All patients underwent CMR within 1 week of initial presentation and had repeat CMR imaging at 3-8 months follow up. CMR was performed on a 1.5 T Siemens scanner. CMR analysis was performed using CVI42 (version 5.11.4, Circle Cardiovascular Imaging Inc., Alberta Canada). Patients were excluded if they did not undergo CMR or did not have a follow up CMR. Initial and follow up CMR data for each patient were reviewed and compared using paired Student t-test. Statistical significance was defined as a p < 0.05. Statistical analysis was performed using SPSS 27 (SPSS Inc., Chicago, IL).”
EXCERPTS FROM RESULTS/CONCLUSION: “A total of 35 patients with the diagnosis of myopericarditis associated with Pfizer COVID-19 mRNA vaccine are followed at our institution. Twelve patients were excluded as they never had CMR due to delayed presentation after initial symptoms resolved or admission to other centers. Six patients were excluded as they did not have a follow up CMR, either because they followed up out of state or a study is still pending. One patient was excluded as initial CMR was performed 3 weeks after presentation. Sixteen patients who had both acute phase and follow-up CMR available for review comprised the final cohort. This group had a median age of 15 years (range, 12-17), were mostly male (n=15, 94%), white and non-Hispanic (n= 14, 88%). One patient was Asian and one patient was American Indian. Median time to presentation from the second dose of the Pfizer COVID-19 mRNA vaccine was 3 days (range 2-4 days). All patients had chest pain. The most common other presenting symptoms were fever (n=6, 37.5%) and shortness of breath (n=6, 37.5%). All patients had elevated serum troponin levels (median 9.15 ng/mL, range 0.65-18.5, normal < 0.05 ng/mL). Twelve patients had c- reactive protein (CRP) measured with median value 3.45 mg/dL, range 0-6.5 mg/dL, normal < 0.08 mg/dL.”
“Ten (62.5%) patients had an abnormal electrocardiogram (ECG), with the most common finding being diffuse ST segment elevation. All patients had an echocardiogram on admission; 14/16 patients had normal left ventricular (LV) systolic function; two patients demonstrated mildly reduced LV systolic function with no dilation. Left ventricular ejection fraction (LVEF) for these two patients was 45% and 53% (normal > 55%). Median left LVEF was 59% (range 45-69%). No patients had pericardial effusion.”
“We previously reported 15 patients with clinically suspected SARS-CoV-2 mRNA vaccine induced myopericarditis. All patients had an abnormal CMR, with edema and or LGE in addition to clinical symptoms and troponin elevation, and some had abnormal ECG or echocardiogram. We have since established a clinical protocol for serial CMR performance in these patients consistent with the 2021 American Heart Association (AHA) statement that stressed the risk of sudden cardiac death, particularly with exercise, while active inflammation is present… our patients were restricted from exercise on discharge. Repeat CMR was performed within 3-6 months to guide next clinical decision-making steps; timing was modified in some individuals based on scanner accessibility and safety precautions during the COVID-19 pandemic. Although symptoms were transient and most patients appeared to respond to treatment (soley with NSAIDS), we demonstrated persistence of abnormal findings on CMR at follow up in most patients, albeit with improvement in extent of LGE.”
“In a cohort of adolescents with COVID-19 mRNA vaccine-related myopericarditis, a large portion have persistent LGE abnormalities, raising concerns for potential longer-term effects. Despite these persistent abnormalities, all patients had rapid clinical improvement and normalization of echocardiographic measures of systolic function. For patients with short acute illness, no dysfunction demonstrated by echocardiogram at presentation and resolution of symptoms at follow up, return to sports was guided by normalization of CMR alone. In patients with persistent CMR abnormalities we performed exercise stress testing prior to sports clearance per myocarditis recommendations. We plan to repeat CMR at 1 year post-vaccine for our cohort to assess for resolution or continued CMR changes.”
“The CDC notes that even though the absolute risk for myopericarditis following mRNA COVID-19 vaccine is small, the relative risk is higher for particular groups, including males 12-39 years of age. Some studies have suggested that increasing the interval between the first and second dose may reduce the incidence of myopericarditis in this population. These data led to an extension in CDC recommended dosing interval between dose 1 and dose 2 to 8 weeks. Further follow up assessment and larger multicenter studies are needed to determine the ultimate clinical significance of persistent CMR abnormalities in patients with post COVID-19 vaccine myopericarditis.”
8. The Journal of the American Medical Association (JAMA) (Published January 25, 2022)
TITLE: Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021
METHODS: “Descriptive study of reports of myocarditis to the Vaccine Adverse Event Reporting System (VAERS) that occurred after mRNA-based COVID-19 vaccine administration between December 2020 and August 2021 in 192 405 448 individuals older than 12 years of age in the US; data were processed by VAERS as of September 30, 2021.”
CONCLUSION: “In this descriptive study of 1626 cases of myocarditis in a national passive reporting system, the crude reporting rates within 7 days after vaccination exceeded the expected rates across multiple age and sex strata. The rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).”
“Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men. This risk should be considered in the context of the benefits of COVID-19 vaccination.”
9. JAMA Cardiology (Published June 29, 2021)
TITLE: Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination
METHODS: “All patients referred for cardiovascular magnetic resonance imaging at Duke University Medical Center were asked to participate in a prospective outcomes registry. Two searches of the registry database were performed: first, to identify patients with acute myocarditis for the 3-month period between February 1 and April 30 for 2017 through 2021, and second, to identify all patients with possible vaccine-associated myocarditis for the past 20 years. Once patients with possible vaccine-associated myocarditis were identified, data available in the registry were supplemented by additional data collection from the electronic health record and a telephone interview.”
CONCLUSION: “In this study of 7 patients with acute myocarditis, 4 occurred within 5 days of COVID-19 vaccination between February 1 and April 30, 2021. All 4 patients had received the second dose of a messenger RNA (mRNA) vaccine, presented with severe chest pain, had biomarker evidence of myocardial injury, were hospitalized, and had cardiac magnetic resonance imaging findings typical of myocarditis.”
“In the 3-month period between February 1 and April 30, 2021, 7 patients with acute myocarditis were identified, of which 4 occurred within 5 days of COVID-19 vaccination. Three were younger male individuals (age, 23-36 years) and 1 was a 70-year-old female individual. All 4 had received the second dose of an mRNA vaccine (2 received mRNA-1273 [Moderna], and 2 received BNT162b2 [Pfizer]). All presented with severe chest pain, had biomarker evidence of myocardial injury, and were hospitalized. Coincident testing for COVID-19 and respiratory viruses provided no alternative explanation. Cardiac magnetic resonance imaging findings were typical for myocarditis, including regional dysfunction, late gadolinium enhancement, and elevated native T1 and T2.”
“In this study, magnetic resonance imaging findings were found to be consistent with acute myocarditis in 7 patients; 4 of whom had preceding COVID-19 vaccination. Further investigation is needed to determine associations of COVID-19 vaccination and myocarditis.”
10. JAMA Cardiology (Published April 20, 2022)
TITLE: SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents
METHODS: “Four cohort studies were conducted according to a common protocol, and the results were combined using meta-analysis. Participants were 23 122 522 residents aged 12 years or older. They were followed up from December 27, 2020, until incident myocarditis or pericarditis, censoring, or study end (October 5, 2021). Data on SARS-CoV-2 vaccinations, hospital diagnoses of myocarditis or pericarditis, and covariates for the participants were obtained from linked nationwide health registers in Denmark, Finland, Norway, and Sweden.”
RESULTS/CONCLUSION:
“Among 23 122 522 Nordic residents (81% vaccinated by study end; 50.2% female), 1077 incident myocarditis events and 1149 incident pericarditis events were identified. Within the 28-day period, for males and females 12 years or older combined who received a homologous schedule, the second dose was associated with higher risk of myocarditis, with adjusted IRRs of 1.75 (95% CI, 1.43-2.14) for BNT162b2 and 6.57 (95% CI, 4.64-9.28) for mRNA-1273. Among males 16 to 24 years of age, adjusted IRRs were 5.31 (95% CI, 3.68-7.68) for a second dose of BNT162b2 and 13.83 (95% CI, 8.08-23.68) for a second dose of mRNA-1273, and numbers of excess events were 5.55 (95% CI, 3.70-7.39) events per 100 000 vaccinees after the second dose of BNT162b2 and 18.39 (9.05-27.72) events per 100 000 vaccinees after the second dose of mRNA-1273. Estimates for pericarditis were similar.”
“Results of this large cohort study indicated that both first and second doses of mRNA vaccines were associated with increased risk of myocarditis and pericarditis. For individuals receiving 2 doses of the same vaccine, risk of myocarditis was highest among young males (aged 16-24 years) after the second dose. These findings are compatible with between 4 and 7 excess events in 28 days per 100 000 vaccinees after BNT162b2, and between 9 and 28 excess events per 100 000 vaccinees after mRNA-1273. This risk should be balanced against the benefits of protecting against severe COVID-19 disease.”
11. Tropical Medicine and Infectious Diseases (Published August 19, 2022)
TITLE: Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents
METHODS: “This prospective cohort study focused on adolescent students from Kong Thabbok Upatham Changkol Kho So Tho Bo School and Wachirathamsatit School who received a second dose of the BNT162b2 mRNA COVID-19 vaccine. The study included subjects who were: (1) aged 13–18 years; (2) male or female; and (3) had received the first dose of the BNT162b2 mRNA COVID-19 vaccine without serious adverse event. Patients who had a history of cardiomyopathy, tuberculous pericarditis or constrictive pericarditis, and severe allergic reaction to the COVID-19 vaccine were excluded from the study. Laboratory tests included cardiac biomarkers (troponin-T, creatine kinase-myocardial band (CK-MB)), ECG, and echocardiography at three clinical visits (baseline, Day 3, Day 7, and Day 14 (optional for subjects with cardiac manifestation)) after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine. Participant data, including demographic data, clinical presentation, and laboratory findings, were recorded in a pre-defined case record form.”
CONCLUSION: “This study focuses on cardiovascular manifestation, particularly myocarditis and pericarditis events, after BNT162b2 mRNA COVID-19 vaccine injection in Thai adolescents. This prospective cohort study enrolled students aged 13–18 years from two schools, who received the second dose of the BNT162b2 mRNA COVID-19 vaccine. Data including demographics, symptoms, vital signs, ECG, echocardiography, and cardiac enzymes were collected at baseline, Day 3, Day 7, and Day 14 (optional) using case record forms. We enrolled 314 participants; of these, 13 participants were lost to follow-up, leaving 301 participants for analysis. The most common cardiovascular signs and symptoms were tachycardia (7.64%), shortness of breath (6.64%), palpitation (4.32%), chest pain (4.32%), and hypertension (3.99%). One participant could have more than one sign and/or symptom. Seven participants (2.33%) exhibited at least one elevated cardiac biomarker or positive lab assessments. Cardiovascular manifestations were found in 29.24% of patients, ranging from tachycardia or palpitation to myopericarditis. Myopericarditis was confirmed in one patient after vaccination. Two patients had suspected pericarditis and four patients had suspected subclinical myocarditis. In conclusion, Cardiovascular manifestation in adolescents after BNT162b2 mRNA COVID-19 vaccination included tachycardia, palpitation, and myopericarditis. The clinical presentation of myopericarditis after vaccination was usually mild and temporary, with all cases fully recovering within 14 days. Hence, adolescents receiving mRNA vaccines should be monitored for cardiovascular side effects. Clinical Trial Registration: NCT05288231.”
“In this observational study, clinically suspected myopericarditis was temporarily associated with the BNT162b2 mRNA COVID-19 vaccine in a small proportion of adolescent patients. Chest pain is an alarming symptom in patients receiving BNT162b2 mRNA COVID-19 vaccination, especially a second dose of BNT162b2. The risk for these symptoms was found to be higher than reported elsewhere. The adverse cardiovascular manifestations observed in this adolescent cohort were both mild and transient.”
From Jessica Rose’s paper, submitted to Current Problems in Cardiology, with contributions from Dr. Peter McCullough. NOTE: Elsevier, the publisher of the journal, has taken the paper down out of PubMed. (The paper is still cited in the National Library of Medicine according to Dr. McCullough.) Rose and McCullough are currently suing Elsevier for withdrawing the paper.
12. Current Problems in Cardiology [Temporarily Withdrawn by Elsevier]
TITLE: A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products
METHODS: “To analyse the VAERS data set the Language and Environment for Statistical Computing, R, was used. The VAERS data set is available for download (https://vaers.hhs.gov/data/datasets) in three separate comma-separated values (csv) files representing i) general data for each report; ii) the reported AEs or ‘symptoms’, and iii) vaccine data including vaccine manufacturer and lot number, for each report. The VAERS dataset is updated approximately once a week and the uploaded set is approximately one week behind the reports. Upon individual reporting of vaccine side effects or adverse events, a VAERS ID number is provided to the individual to preserve confidentiality, and a detailed description of the side effects are transcribed along with the individual’s age, residence by state, past medical history, allergies and gender and many other details. In addition, the vaccine lot number, place of vaccination and manufacturer details are included in the report. In order to maximize the input variables for my analysis, the three files were merged by VAERS ID that is included as a linking variable in all three files. The merged data set comprises data collected pertaining to all reported AEs associated with BNT162b2, mRNA-1273, and Ad26.COV2.S products: the three primary vaccine manufacturers responsible for nCoV-2019 products currently being administered in the U.S. Data was sorted according to vaccine type (data reported for COVID-19) and relevant variables were sorted including VAERS ID, AEs, age, gender, state, vaccination date, date of death, incident of death, dose series, treatment lot number, treatment manufacturer, hospitalizations, emergency department visits and onset date of AEs. Myocarditis as a standalone AE was extracted by keyword and cardiac events were grouped by extracting multiple keywords according to MedDRA nomenclature. Statistical analysis was done using the Student’s t-Test to determine statistically significant differences between ages in the myocarditis AE. Skewing in distribution of data was tested using Pearson’s Skewness Index, I, which is defined as I = (mean-mode)/standard deviation. The data set is significantly skewed if |I|≥1.”
CONCLUSION: “These data are derived from a rushed, non-FDA-approved, ongoing investigational product roll-out, and our conclusions are thus limited by the information at hand. In addition to the 12-15-year-old age group data being very early, it is vital to acknowledge that these reports represent a fraction of the actual total. Thus, due to both the problems of under-reporting and the known lag in report processing, this analysis reveals a strong signal from the VAERS data that the risk of suffering CIRM – especially males is unacceptably high. Again, children are not a high-risk group for COVID-19 respiratory illness, and yet they are the high-risk group for CIRM.
Efficacy of these products needs to be assessed by immunological assays and long-term studies are required, while safety needs to be evaluated by rigorous clinical, laboratory and imaging assessments of severe reported adverse events such as CIRM. Autopsies should be done in cases of cardiovascular-related deaths temporally associated with COVID-19 injectables. It is reasonable to use the precautionary principle in this particular setting since an alarming number of reports are coming from young males between the ages of 12 and 15. Boys of these ages should be carefully monitored for warning signs of myocarditis which many may pass off such as pallor, chest pain, shortness of breath or lethargy, following dose 1 with the aim of seeking prompt evaluation and avoiding dose 2.”
Below author Jessica Rose notes that: “What we found…me and Dr. Peter McCullough, co-authors on this paper, we found—the most prevalent finding—was that 19 times above background rate myocarditis was seen in children age 12 to 15. And this is striking….If it was five times it would still be crazy, but 19 times is…not something you can ignore. And the CDC and FDA didn’t. But they are [understating] it. They didn’t take it seriously enough and are still kind of brushing it aside as a non-issue. For anyone wondering if COVID-induced myocarditis rates are higher or even equal to the injection-induced myocarditis rates, they’re not. You can look that up….And way more so if [people] are male, and if they are young.”
From Voice for Science and Solidarity: “Dr. Jessica Rose is a Canadian researcher with a Bachelor’s Degree in Applied Mathematics and a Master’s degree in Immunology from Memorial University of Newfoundland. She also holds a PhD in Computational Biology from Bar Ilan University and 2 Post Doctoral degrees: one in Molecular Biology from the Hebrew University of Jerusalem and one in Biochemistry from the Technion Institute of Technology.”
Dr. Peter McCullough explains how he and Jessica Rose’s peer-reviewed VAERS analysis was inexplicably retracted by the publisher
“Elsevier is going to be absolutely slaughtered on this. I can make that a promise.”
Full video https://t.co/a6aiSrwIXX pic.twitter.com/KDr0dDpBvZ— Mr. M (@Mr_Mackei) December 24, 2021
Opinion: Pediatric Reports (Published September 13, 2021)
TITLE: COVID 19 Vaccine for Adolescents. Concern about Myocarditis and Pericarditis
ABSTRACT: The alarming onset of some cases of myocarditis and pericarditis following the administration of Pfizer–BioNTech and Moderna COVID-19 mRNA-based vaccines in adolescent males has recently been highlighted. All occurred after the second dose of the vaccine. Fortunately, none of patients were critically ill and each was discharged home. Owing to the possible link between these cases and vaccine administration, the US and European health regulators decided to continue to investigate the potential causal relationship between COVID-19 mRNA vaccines and myocarditis. In any case, none of the patients fulfilled the criteria for multi-system inflammatory syndrome or Kawasaki-like disease and there was no evidence of acute SARS-CoV-2 infection.
CONCLUSION: “Certain characteristics are pointing toward a “rare, but real” clinical entity of myocarditis/pericarditis following COVID-19 vaccination. First, the events occur within days since vaccination. Second, they tend to be more common in males and in younger people. Third, the number of adverse events is greater than the so-called “standard myocarditis incidence rate”. We feel that there should be at least a year of study and follow up from vaccination in clinical trials, the amount of data typically essential for full approval, instead of the 2 months required for emergency use authorization.
At the moment, three questions remain open and unanswered, namely: why is myocardium a side effect? Why are adolescent males affected the most? Why is the onset after the second dose of m-RNA vaccine?”
13. Medical Archive (Not Yet Published in a Peer Reviewed Journal) (Posted online on December 18, 2022)
TITLE: Booster Vaccination with SARS-CoV-2 mRNA Vaccines and Myocarditis Risk in Adolescents and Young Adults: A Nordic Cohort Study of 8.9 Million Residents
METHODS: “Cohort participants [n=8.9 million] were followed until an inpatient diagnosis of myocarditis, loss to follow-up, or end of study (latest data availability in each country), whichever occurred first. In each of the four countries, Poisson regression was used to estimate adjusted incidence rate ratios (IRRs) of myocarditis, with associated 95% confidence intervals (CIs), according to vaccination status. Country-specific results were combined in meta-analyses.”
CONCLUSION: “A total of 8.9 million residents were followed for 12,271,861 person-years. We identified 1533 cases of myocarditis. In 12-to-39-year-old males, the 28-day acute risk period following the third dose of BNT162b2 or mRNA-1273 was associated with an increased incidence rate of myocarditis compared to the post-acute risk period 28 days or more after a second homologous dose (IRR, 2.08 [95% CI, 1.31 to 3.33] and 8.89 [95% CI, 2.26 to 35.03], respectively). The corresponding incidence rates following the third dose of BNT162b2 and mRNA-1273 were 0.86 and 1.95, respectively, within 28 days of follow-up among 100,000 individuals.”
“Our results suggest that a booster dose is associated with increased myocarditis risk in male adolescents and young male adults.”

14. Journal of Clinical Medicine (Published October 14, 2022)
TITLE: Hospitalised Myocarditis and Pericarditis Cases in Germany Indicate a Higher Post-Vaccination Risk for Young People Mainly after COVID-19 Vaccination
METHODS: “Hospital renumeration data from Germany based on the International Classification of Diseases (ICD-10) were analysed. Myocarditis was assumed when the principal diagnosis was coded as I40.8 (other acute myocarditis), I40.9 (acute myocarditis, unspecified), or I51.4 (myocarditis, unspecified); pericarditis was assumed when the principal diagnosis was coded as I30.9 (acute pericarditis, unspecified). An adverse reaction to COVID-19 vaccines was assumed when the secondary diagnosis was coded as U12.9 (COVID-19 vaccines causing adverse effects in therapeutic use, unspecified). An adverse reaction to any vaccine including a COVID-19 vaccine was assumed when the secondary diagnosis was coded as Y59.9 (complications due to vaccines or biological substances) or T88.1 (other complications following immunisation, not classified elsewhere).”
CONCLUSION: “In 2019 and 2020, there were no or only very few cases (<4) of myocarditis or pericarditis described as adverse events after any type of vaccination (Y59.9 or T88.1). None of them required intensive-care treatment. In 2020, 32 cases of myocarditis or pericarditis were hospitalised as COVID-19 patients (U07.1), with 15 of them requiring intensive-care treatment. In 2021, the number of hospitalised myocarditis or pericarditis cases among juveniles (10–17 years) had increased from 270 (2019) and 196 (2020) to 506 (2021). In total, 11 cases (2.2%) were associated with COVID-19, 160 cases (31.6%) were associated with a COVID-19 vaccine or vaccination in general, and 32 cases required intensive-care treatment. Similar results were found for young adults (18–29 years).”
SWEDEN AND FINLAND HALT MODERNA “VACCINE” FOR PEOPLE UNDER 30
Not only are there compelling studies evincing the COVID-19 “vaccines” are more likely to induce myocarditis than the disease itself in those under 40, Sweden and Finland have already withdrawn the use of Moderna in those under 30.
As Reuters reported on October 7, 2021, Finland joined Sweden in limiting the Moderna “vaccine” for those under 30. (Note that according to a Reuters report on October 8, 2021, Denmark said it never stopped vaccination of the young with the Moderna “vaccine.”)
As of January 17, 2022, the Finnish Institute for Health and Welfare says that “The Moderna Spikevax vaccine is not currently offered to boys and men under 30 years of age in Finland, as according to a Nordic follow-up study, the highest number of myocarditis cases has been observed in young men after coronavirus vaccination.”
On October 27, 2021, Reuters reported that Sweden’s health ministry had extended the pause on the use of the Moderna “vaccine” for both men and women born 1991 or later.
Also note that according to a November 9, 2021 report by Reuters, France began to recommend against the use of Moderna in males under 30. Germany, likewise, has recommended against the use of the Moderna “vaccine” for those under 30.



Recent Comments